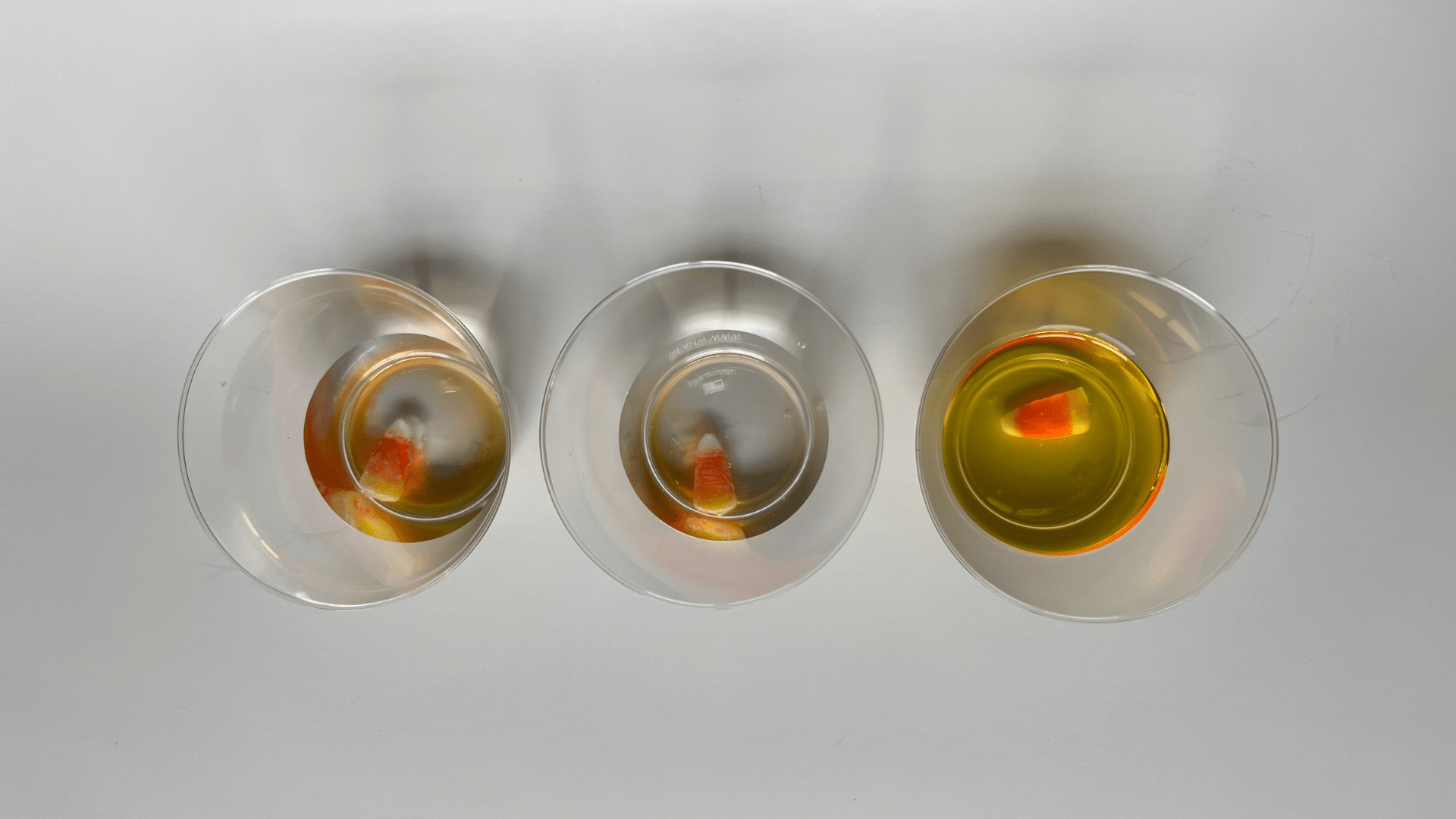Candy Corn Chemistry
Candy corn is a “mellow creme” meaning that is made from corn syrup and sugar that has a marshmallow-like flavor. This is injected into a mold in three colors (white, orange, and yellow) and then allowed to sit for 24 hours until it hardens. After it hardens, the candy corn is coated with a confectioners glaze of oil and edible wax to make it shiny. All matter is made up of molecules. Molecules are held together by the bonding of positive and negative charges. To dissolve something, the molecules in a solid and the molecules in a liquid need to be attracted to each other using these positive and negative charges. If the molecules are not attracted to each other, the solid will not dissolve. The sugar in the candy corn and the water both have positive and negative charges that attract each other. Eventually, the water will break all the bonds between all the sugar molecules in the candy corn causing it to completely dissolve. The vinegar molecules can’t attract the sugar molecules as well, so the candy corn doesn’t dissolve well in vinegar. The oil molecules have no positive or negative charges, so the candy corn does not dissolve at all in oil.